Medicines & Treatment Safety
LMVO - Drug safety at the heart of our concerns
Through the presidency of the LMVO (Luxembourg Medicines Verification Organisation), we ensure the application of the directives to prevent falsified medicines from entering the legal distribution chain of medicines in Luxembourg.
In practice, the safety of medicines is ensured by two measures. The first security is the unique 2D Data Matrix QR code. It is a code that contains the serial number, the manufacturer's number, the batch number, the box number and the expiration date. This is called serialization.
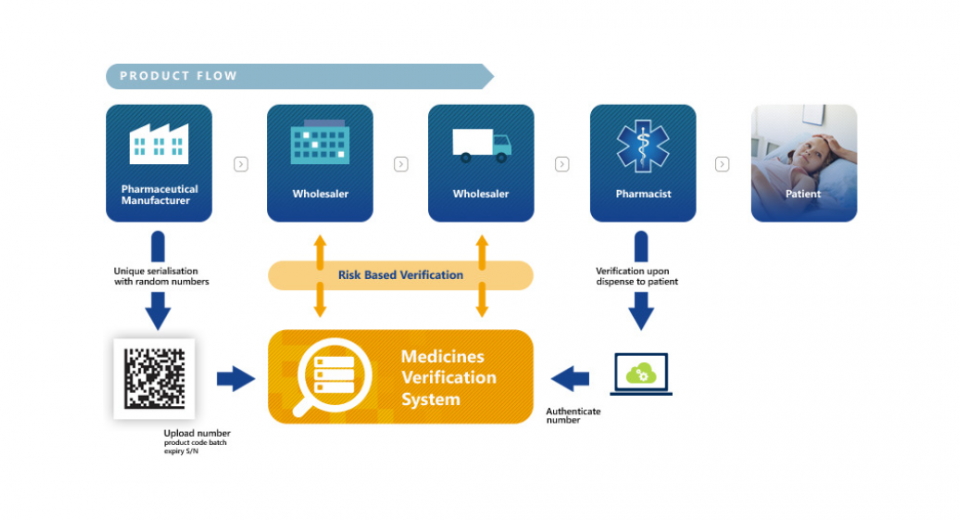
Each box has its own number. When it leaves the company, we put this number in a database and when it arrives at the pharmacy, the pharmacist scans the box. Not only to get the price but also to "decommission" it. This box has been delivered to a patient and this unique code cannot be found on another box for sale. In the event that this happens, an alert is issued because one of the two boxes is a fake and thanks to the traceability, we can trace the two patients concerned in order to identify the fake box among the two.
The system ensures additional and unequalled security for patients by scanning the unique identifier contained in the Data Matrix of each box dispensed and comparing it with the corresponding code entered in the central database when the drug is released into the distribution circuit.
The serialization system completes the existing batch traceability by authenticating each box of medication at the time of dispensing.
The second security feature is that each box of prescription drugs has a tamper-evident seal - a kind of sticker that closes the packaging and cannot be re-sticked - allowing visual verification of the integrity of the box.
This is an unprecedented challenge for all stakeholders, allowing for better control during dispensing and a reduction in public health risks.

Interested in becoming a partner?
To benefit from all of our services, more particularly :
- Participate in committees and commissions, in the different working groups
- Free access to our intranet
- Profit of the organizations networh: exchangres between experts, invitation to formations and exclusive meetings (economical, scientific, industrial, social, legal, RSE, parlementary, european, communication…)
- Being represented and being defended in front of institutions and the public, see the image of drug producing companies valued.
for more detailed information about the adhesion steps, please, contact us by mail contact@iml.lu .